Background: 60% of Diffuse large B-cell lymphoma (DLBCL) are cured with R-CHOP. Treatment options for relapsed/refractory (R/R) patients (pts) not eligible to high-dose chemotherapy and autologous stem cell transpntation (ASCT) or CAR-T are limited with short-lasting efficacy. The PI3-K inhibitor copanlisib showed activity in B-cell lymphomas including DLBCL. We reported the results of the phase II single arm multicenter FIL Copa-RB trial which investigated the combination copanlisib plus rituximab and bendamustine (copa-RB) in transplant-ineligible R/R DLBCL.
Methods: Patients with R/R DLBCL previously treated with 1-3 prior lines, not eligible to ASCT and CAR-T were enrolled to receive 6 cycles of copa-RB q28 days (induction treatment schedule: copanlisib 60 mg i.v. days 1,8,15. Rituximab 375 mg/m2 i.v. day 1, Bendamustine 90 mg/m2 i.v. days 1,2). Patients with at least stable disease continued with a maintenance phase up to 12 q28 day cycles of Copanlisib (60 mg i.v. days 1,15). The primary end point was 12-month progression free survival (PFS) with an expected improvement from 20% to 35% and a calculated sample size of 81 pts.
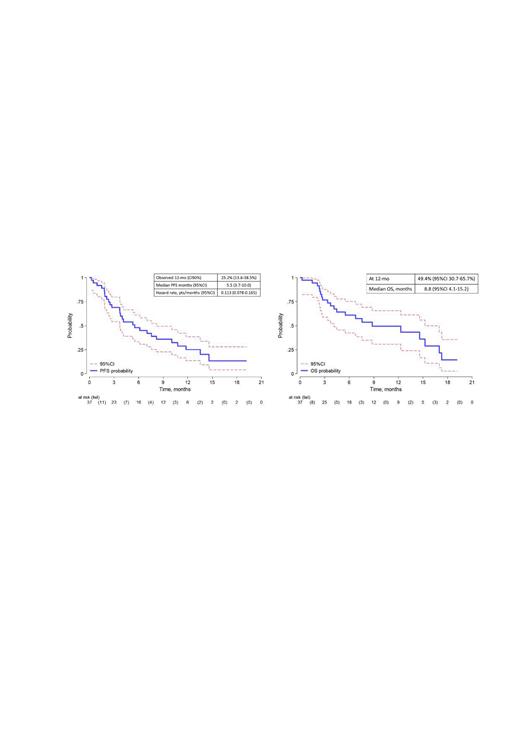
Results: From 11/2019 to 7/2022, 37 pts were enrolled. Median age was 76 years (68-87), 23 (62%) were at IPI ≥3, 25 (68%) had stage IV, 23 (62%) and 14 (38%) received 1 and 2 prior lines and 35% were refractory to the last treatment. The best overall response rate (ORR) was 51%, with 22% CR. With a median follow-up of 12 months, the 12-mo PFS was 25.2% (95%CI:13.6-38.5%) and median PFS 5.5 months (95%CI:3.7-10.0). The 12-mo overall survival (OS) was 49.4% (95%CI:30.7-65.7%) (fig. 1). Nine patients were alive and in continuous response at time of the data cut-off. 91% of the patients experienced grade > 2 adverse events (AE): neutropenia (58%), infections (28%), gastrointestinal (19%), skin (16%) and thrombocytopenia (13%). SARS-COV-2 infection occurred in 19% with 5 deaths. CMV reactivation occurred in 47%, but without infection. Febrile neutropenia was recorded in 2.8%. AEs led to dose interruptions in 19%.
Since the slow recruitment, the unfavorable safety profile and the emerging of potentially more effective new treatment options in this setting of patient, the study was interrupted prematurely by steering committee decision.
Conclusions: Copa-RB study was conducted in a difficult-to treat R/R DLBCL cohort (elderly, advanced stage) during COVID pandemic that affected its management. Copa-RB induced a good ORR, but it was short lasting with unfavorable safety profile, so the overall activity was modest and did not support further the development of this combination in this setting of patients. A subset of patients had a long-lasting response. A mutational study is ongoing to identify molecular pathways potentially predictive of response to the combination
OffLabel Disclosure:
Tucci:Gentili: Other; Sanofi: Other; Janssen: Other; Takeda: Other; Eli Lilly: Other; Kiowa Kiryn: Other; Beigene: Other. Gini:Takeda: Consultancy; Gentili: Consultancy; Incyte: Consultancy; Roche: Consultancy. Arcari:Janssen, Abbvie, Takeda, Servier: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Vitolo:Bayer: Membership on an entity's Board of Directors or advisory committees; Genmab: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; AbbVie: Other: Lecture Fees; Incyte: Other: Lecture Fees; Janssen: Other: Lecture Fees; Roche: Other: Lecture Fees; Servier: Other: Lecture Fees.
Copanlisib is an OffLabel drug for DLBCL

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal